Which Of The Following Best Represents The Reaction Between Sulfuric Acid And Calcium Hydroxide?
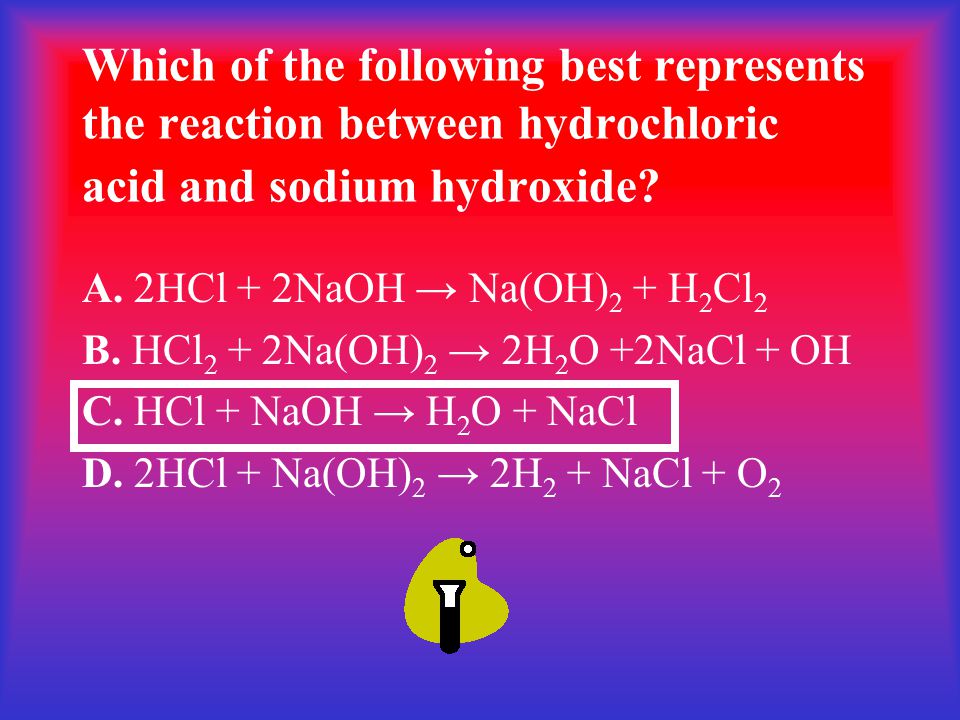
Which of the following best represents the reaction between sulfuric acid and calcium hydroxide?. WORKSHEET- MOLECULAR AND IONIC EQUATIONS Any soluble substance given below is in aqueous solution. The ionic equation for any acid reacting with any base is. When sulfuric acid reacts with sodium hydroxide sodium sulfate and water form.
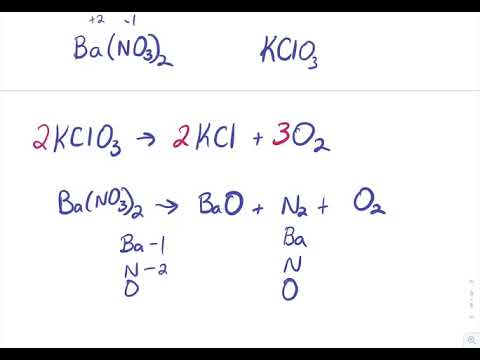
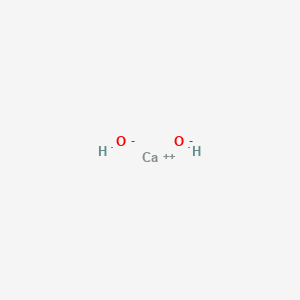
Sulfuric acid is represented as H₂SO₄ and calcium hydroxide is represented as CaOH₂. Sulphuric acid Calcium hydroxide Calcium sulphate Water H 2 S O 4 2 H S O 4 2 As H 2 S O 4 gives H in water it is an acid Arrhenius Theory. Step-3Now balance Na atom firstIn the left hand sidewe have one Na but in the right sidewe have two NaSo to balance Na we need to put 2 before NaOH in the left side.
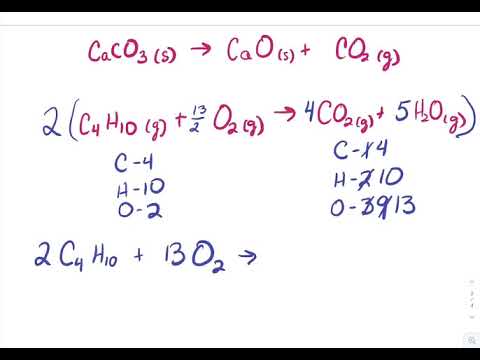
Haq OH-aq -- H2Ol The H2SO4 provides the H ions. Concentrated sulfuric acid like any type of acid can be most easily neutralized by combining it with a material that has a basic nature on the pH scale with calcium carbonate CaCO 3 and calcium hydroxide CaOH 2 being two of the compounds most frequently used. H2SO4 CaOH2 CaSo4 2H2O One molecule each of sulfuric acid and calcium hydroxide react to give one molecule of.
C O2 CO2. F H2SO4 CaOH2 CaSO4 H2O G HSO4 CaOH CaSO4 H2O H H2SO4 CaOH2 CaSO4 2H2O J H2SO4 2CaOH2 2CaSO4 3H2O. Ca OH 2H2SO4CaSO42H2O Calcium Hydroxide and Sulfuric Acid are mixed.
The OH- ions come from the sodium hydroxide. The balanced chemical equation for the reaction between Sulphuric Acid H2SO4 and Calcium Hydroxide Ca OH2 will be as follows. Sulfuric acid is represented as H₂SO₄ and calcium hydroxide is represented as CaOH₂.
Of different atoms in both sides reactants and products. Get the detailed answer. Which of the following is the correct word equation for the reaction described below.
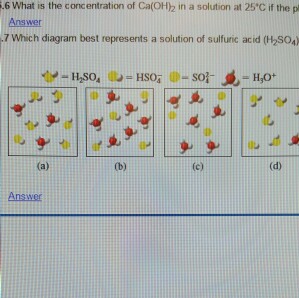
Write a balanced equation for the following neutralization reaction. Which of the following best represents the reaction between sulfuric acid and calcium hydroxide.
Sulfuric acid is represented as H₂SO₄ and calcium hydroxide is represented as CaOH₂.
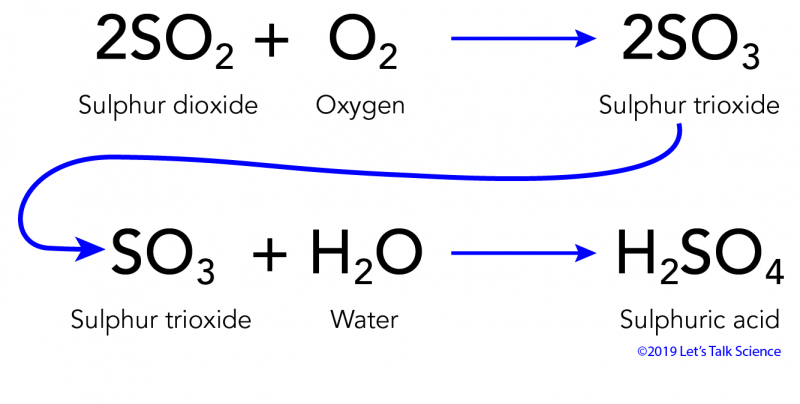
Theoreticallythe reaction between Calcium Hydroxide and Sulfuric Acid should take place as an acid-base reaction forming a salt and water. F H2SO4 2CaOH2 2CaSO4 3H2O g H2SO4 CaOH2 CaSO4 2H2O h HSO4 CaOH CaSO4 H2O j. Of different atoms in both sides reactants and productsThe only choice that represent the reactants correctly and apply the law of conversation of mass is. H₂SO₄ 2NaOH -----Na₂SO₄ H₂O. Which is an example of a synthesis reaction. To balance this reaction means we need to equalize the number of these above atoms and polyatomic ion. To balance a chemical reaction. WORKSHEET- MOLECULAR AND IONIC EQUATIONS Any soluble substance given below is in aqueous solution. Which of the following is not an oxidation reduction reaction.
Sulfuric acid is represented as H₂SO₄ and calcium hydroxide is represented as CaOH₂. H2SO4 Ca OH2 Ca SO4 2 H2O One molecule of Sulphuric Acid and. Which of the following best represents the reaction between sulfuric acid and calcium hydroxide. Which of the following best represents the reaction between sulfuric acid and calcium hydroxide. Which of the following best represents the reaction between sulfuric acid and calcium hydroxide. The balanced chemical equation for the reaction between Sulphuric Acid H2SO4 and Calcium Hydroxide Ca OH2 will be as follows. To balance a chemical reaction.



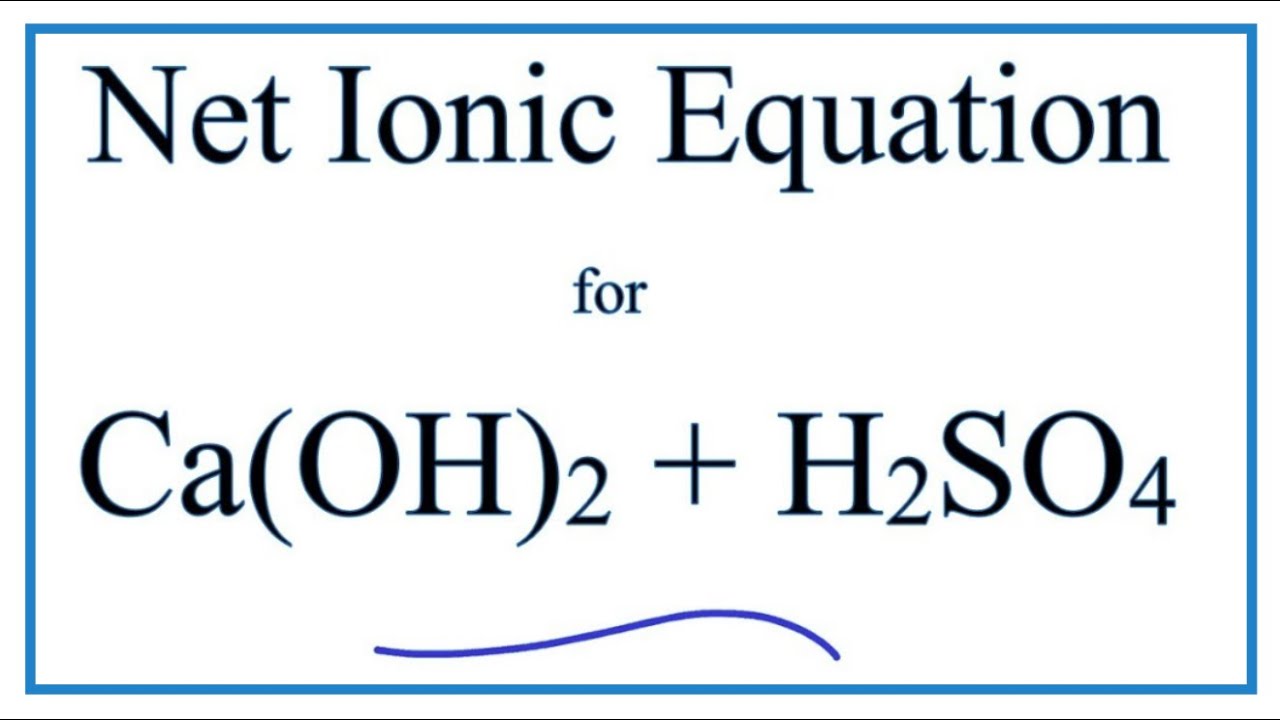



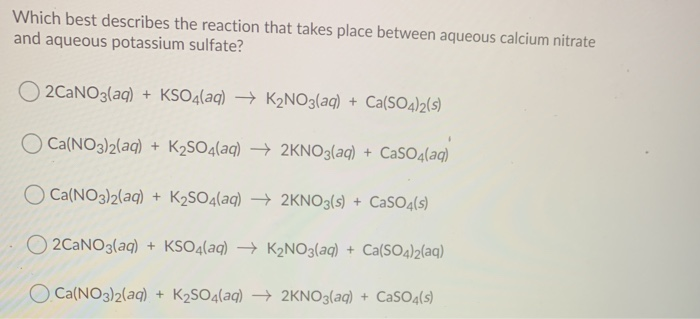






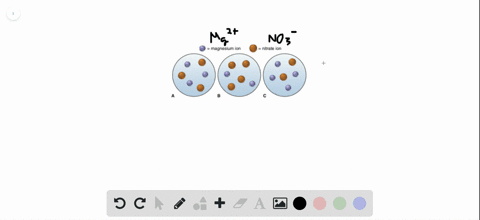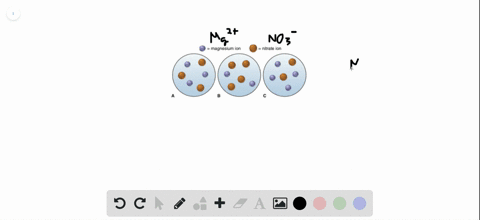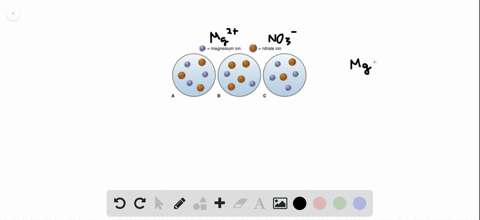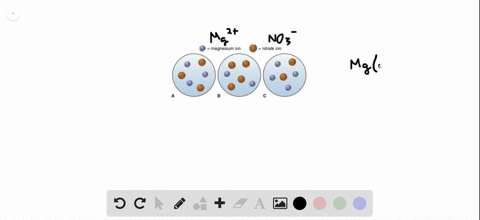



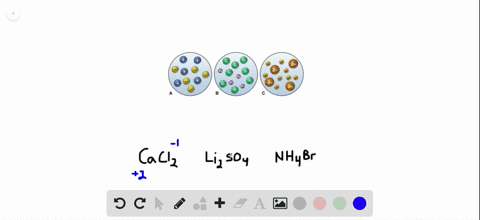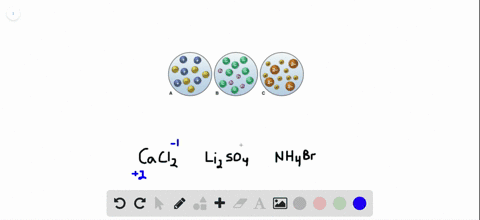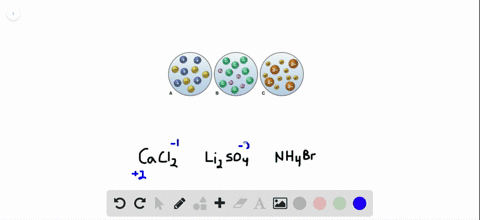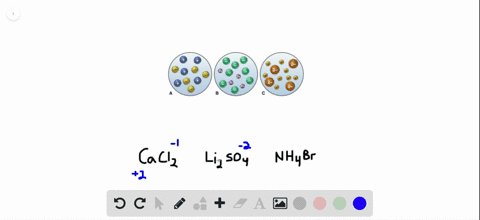


Post a Comment for "Which Of The Following Best Represents The Reaction Between Sulfuric Acid And Calcium Hydroxide?"